ANSWER KEY
TO MIDTERM PRACTICE QUESTIONS 2015
printer friendly pdf
1a.
Y = visible light part of
spectrum 1b.
Z =
infrared part of spectrum 1c.
X = UV part of spectrum
2.
(c) since there are
3 electrons
depicted in the atom, and it is neutral, then there have to be 3 protons in the
nucleus. [see p 13 in Class Notes for a review]
3.
(d) when energy
is absorbed by an electron, it leaps to a higher energy level.
Remember that an electron is MATTER,
while a photon is ENERGY -- the MATTER does the absorbing and emitting, while
the PHOTON of energy is the thing that IS absorbed or emitted.
4. FALSE
We haven't yet talked much about the ozone
hole -- this is NOT how it works or why it is important so the statement is
false. But have you pieced together in your mind how the Earth DOES cool??
ANSWER: The earth cools by radiating a great deal of its infrared
(IR) radiation out to back out to space through the IR Atmospheric Window!
5.
TRUE 6. (d)
see p. 43 in SGC-E-Text Chapter 3: section on Planetary Energy Balance
7. (a)
this is just the inverse of the "mantra quote" in the box on the bottom of p 21 of Class
Notes (below the cartoon with the rope being shaken): "The shorter the wavelength the greater the energy & the higher the
frequency"
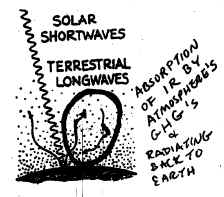
8. (c)
9. (d)
[ See the section on "Physical Causes of
the Greenhouse Effect" on pp 48-50 in SGC-E-Text Chapter 3 for a review of this concept
]
10. (c)
see the
section in SGC-E-Text referred to for #9 above.
11. (b)
see p 33
in Class Notes and the Table 3-2 and 3-3 in SGC-E-Text
12.
(b) Remember
that short wavelengths, high frequency, and high energy all go together, and be
sure you understand that ultraviolet (UV) wavelengths are
shorter than
infrared (IR) wavelengths. Another key thing to remember is that
ONLY
infrared (IR) energy is involved in the Greenhouse Effect!!
13. (d) This one takes
careful reading. There is something wrong in every choice but (d).
Choices (a) and (c) both imply that that long wavelengths and hot temperatures
go together, but you should remember that Wein's Law is an inverse
relationship between wavelength and temperature, so they can't be correct.
Choice (b) describes the relationship between wavelength and radiation intensity
(energy flux), not temperature, hence it is also incorrect. That
leaves choice (d) which is the "mantra" for Wein's Law.
[TEST-TAKING HINT: Note that in Question #13 the phrases
bolded are there
to help you sort through similar sounding phrases.
Look
for clues like this in the real exam and TAKE YOUR TIME so you can sort out the
correct answer logically based on what you know and clues that are given in the
question. ]
14.
(c) Using the logic described
above, only (c) relates wavelength (lambda) with
temperature in an
inverse relationship.
15.
(d) To answer this one correctly you
must understand the underlying principle of absorption curves. Each curve
represents the wavelengths that are absorbed easily by a specific substance
(usually a gas) or group of gases. Not all substances absorb and
emit the same wavelengths of energy, even if the temperature of the substance is
exactly the same. It's true that (a), (b), and (c) are all correct
statements based on the radiation laws, but only (d) gets to the essence of why
different gases have different absorption curves.
16. (c)
both solar and terrestrial
Graph B is the graph of absorption by ozone gas. It shows
that ozone can absorb harmful UV wavelengths (which is why the ozone layer in
the stratosphere is beneficial) and it can also absorb IR wavelengths (which is
why ozone can also be considered a greenhouse gas).
[NOTE: Ozone enters into Global Change
issues in two different ways: (1) as a factor in the ozone layer and the
ozone "hole" issue (which we'll be covering later), and (2) as a greenhouse
gas. These are TWO VERY DIFFERENT ISSUES with very different processes
involved! The key to understanding the difference between the two issues is
in the dual properties of ozone absorption -- the fact that ozone is a
greenhouse gas that absorbs IR radiation is a totally DIFFERENT property than its additional ability to absorb in the UV
part of the spectrum!!!! Don't confuse these two things!!!]
17. (a)
be careful not to get matter and energy mixed up --
photons are electromagnetic energy, protons are particles inside an atom!
18.
(c) Here you have to be familiar
with the wavelengths of maximum emission of energy by both the Sun (0.5
micrometers in the middle of the visible light part of the spectrum) and the Earth (10.0
micrometers in the infrared (IR) part of the spectrum. Choice (d) states things
backward with respect to the greenhouse effect. Choice (e) may sound like
the right answer, but remember that the Sun radiates in ALL wavelengths, not
just visible light.
19. ( b)
20. ( c)
21. (a)
review p 42 in Class Notes
22. (b)
You can figure this one out if you remember that
the shortest wavelengths are the most harmful because they contain the
highest frequency wavelengths of electromagnetic energy. You also need
to remember that the ozone layer absorbs the most harmful wavelengths of UV
radiation (UVC and some UVB), but not UVA. See also Arrows 3 & 4 in the
figure on p. 32 of Class Notes. UVC
radiation is very harmful, and luckily both UVC and most of the UVB are absorbed
by gases in the atmosphere BEFORE they reach the Earth's surface. UVA (and
actually some more harmful UVB) gets through the atmosphere to the surface,
which is why we need sunscreen!
23. Troposphere
24. Stratosphere
25. Mesosphere 26.
Thermosphere
[HINT: if this appears as a multiple choice question, don't get thrown off
by similar sounding, but incorrect choices such as:
tropopause,
stratopause, "menopause" etc. etc.
27. (c) It's not (a) because
atmospheric pressure decreases with altitude (see Fig 3-9a in SGC-E-Text). It's
not (b) because CO2
concentration (as well as the concentration of
all the other GHG's except for ozone) is highest in the
troposphere. It's not (d) because temperature is warmest at the Earth's
surface and then decreases through the troposphere -- this figure shows the
highest values are in the stratosphere.
28. (b)
All the statements correctly describe aspects of a
Radiation Law, but (b) is the only one that relates to the Inverse Square Law
29.
(a)
If you wre paying attentiom during the "Name that Gas" activity, this one will
be easy -- also see the Greenhouse Gas Overview on p 34 of Class Notes.
30.
(a)
convection
is defined as the transfer of energy by means of large-scale movements of matter
(recall that conduction is molecule-to-molecule transfer of energy (with
the molecules not changing position) and that the transfer of energy as LW
(infrared) radiation or SW (UV or visible light) radiation occurs as
electromagnetic waves which can transfer energy without matter.
IMPORTANT: Don't confuse
outgoing IR radiation with rising warm air!
Infrared (IR) is energy, NOT
moving warm air -- the IR energy is NOT sensed as heat or warmth until it is
ABSORBED by something (e.g., greenhouse gas molecules, etc.)
31.
(d) incoming
shortwave energy (UV, visible light) is transferred as photons or pulses of
energy
32. (b)
the sand will heat up faster and get hotter
than the water because it takes less energy (in calories) to change its
temperature. (It will also cool
off faster than the water) Remember that because of its high specific
heat and heat capacity, water takes longer to heat up, but once heated up,
it will hold that heat longer.
Substances with lower specific heats and heat capacities (like air and soil)
respond more readily to changes inputs of energy than water and heat up and
cool off faster.
33. (c)
Figure X implies that
the IR/ longwave (LW) terrestrial radiation is being reflected back to the
Earth's surface. What really happens is that the IR radiation is
absorbed by the Greenhouse
gases (GHGs) in the atmosphere, and then radiated
back down to the surface. As discussed in
class, both Y and Z show the absorption and re-radiation of IR, but Y also has
part of the incoming shortwave (SW)/Solar radiation circled. The
Greenhouse Effect (GHE) involves ONLY
INFRARED radiation, so circling some of the Solar / SW makes Y an incorrect
choice. HINT:
If you are asked to circle the part of the figure that represents the GHE, be
careful that you circle only
the Terrestrial IR/LW radiation part of the figure and not some of the Solar/SW
too!
34. (c)
We learned that a well-designed LED (light emitting diode) bulb can be up to 80
% efficient, which means that the thermal energy lost due to inefficiency will
be 20%. The input of energy to a light bulb is electrical energy.
35.
(a)
The Law of Conservation of
Energy is the same thing as the 1st Law of Thermodynamics ]
36. (d)
Look closely at the figure!
The thermal energy flow is going from
the cold ice cube
to the warm finger and this violates
the 2nd Law of Thermodynamics!
end of multiple choice practice questions
------------------------------------------------------------------------------------------------------------------
SAMPLE ESSAY QUESTION (and a few more
multiple choice too!)
37a. Of the 3 figures shown,
Figure Y displays the best representation of the
Greenhouse Effect (although note that it doesn't represent ALL the pathways
of SW and LW that are discussed on the bottom of page 29 in Class Notes.. )
37b.
(see sketch below )
Be
sure you do NOT circle any of the
SW radiation part of the figure -- the
GREENHOUSE EFFECT involves LW (Infrared) radiation ONLY
 |
37c. (Be sure to explain
WHY X and Z are
incorrect in addition to saying WHY Y
is more accurate.)
Figure X depicts all terrestrial LW radiating
right back out to space;
Figure Z implies that the terrestrial LW is REFLECTED back down to the Earth's
surface -- this is NOT what happens!
Figure Y is more accurate because it
shows
the LW being ABSORBED by gases in the atmosphere and then being RADIATED (not
reflected) back to the Earth's surface.
37d. Here's the definition
given in the Midterm Study Guide (under Topic #5):
"The greenhouse effect is the natural mechanism by which the Earth's surface is
warmed by infrared-absorbing gases (i.e. greenhouse gases) in the atmosphere."
|
NOW,
work on your OWN wording of the concept -- don't just memorize or repeat the
above. Avoid words like
"bounce" or "reflect" whenever you are discussing longwave
infrared radiation! Instead be sure to make the point that the greenhouse
gases absorb IR and then radiate it (emit it) out again. Also, don't get
confused in your words and start talking about the greenhouse gases being
absorbed! The gases do the absorbing and emitting of the IR radiation,
they are NOT absorbed themselves.
38.
(a) troposphere
39. [For the answer,
see p 31-32 in Class Notes and the discussion on the Structure of the
Atmosphere in SGC-E-Text Chapter 3.] The troposphere
decreases in
temperature with height because it is primarily heated
from below by
terrestrial infrared energy radiating upward from the Earth's surface. The
stratosphere increases in temperature with height because the ozone layer
is in the stratosphere and the higher you go in the stratosphere the more
incoming UV there is to be absorbed by ozone (and oxygen). When these gas
molecules absorb the high energy UV radiation, they are energized and move
faster ("jiggle" more) and hence the atmosphere warms up at this level.
(Note however that the air in the stratosphere is much less dense than the air
in the troposphere.)
40. This question is one that asks you to apply
concepts you've learned to a less familiar topic: the Kramer Junction solar
plant in the Saved by the Sun video.
(a)
ENERGY is transferred from X-to-Y (from the
SUN to the SYNTHETIC OIL in the red tubes) by
RADIATION
(b) When water in the Solar Super-heater
vat boils
instantly, the Y-to-Z heat transfer that occurs
can be described
as the transfer of
SENSIBLE HEAT
in
the SYNTHETIC OIL to
LATENT HEAT in the STEAM .
( The terms in red were in your SGC readng, but have not been discussed in class
yet )
NOTE: There are lots of energy transfers
going on this solar thermal plant!!
I selected some of the most basic ones to ask about in this question.
Do you have a feel for how does this kind of solar
energy technology differs from a photovoltaic cell?
ANSWER:
a
photovoltaic cell involves photons from the sun being absorbed by a silicon
layer and "knocking loose" the electrons from their atoms in the silicon
layer so that electricity can be generated. (If you are curious and interested in
learning more details for your Linking-to-Life Project, see:
http://www.pbs.org/wgbh/nova/tech/how-solar-cell-works.html and
http://www.pbs.org/wgbh/nova/tech/solar-tech.html
41. (see sketch below)
(If you get a question like this, be sure your follow all the directions and
LABEL your sketch (as shown below) in addition to just drawing in the
answer.)
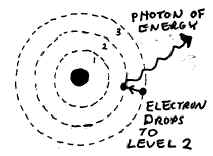
NOTE: Be sure that you depict the
ELECTRON
leaping between energy levels, NOT a photon! The electron emits or
absorbs a photon of energy, not the other way around. Also be sure you
show the photon being EMITTED if the electron leaps to a LOWER energy level.
(Or, if the question asks you to show what happens when a photon is ABSORBED,
show that the electron leaps to a higher energy level.)
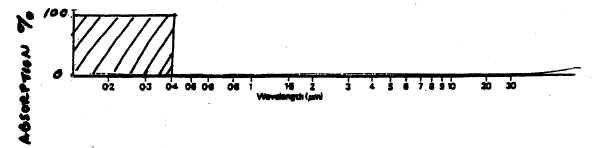
42. The
sketch below represents a hypothetical atmosphere in which ALL
UV is absorbed, while all VISIBLE & IR radiation is transmitted, or allowed to
get through the atmosphere:
|
43. To
answer this you need to know that Energy
Efficiency = work done/ energy used
The work done is the production of Electricity and the
energy used is the Energy from coal, therefore:
(a)
Energy Efficiency = 600 MW /
1200 MW = 50%
(c) Energy
Efficiency = 800 MW / 1200 MW = 66.7%
|
(b)
Your sketch should look
like something this:
 |
|
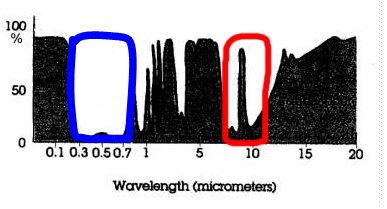
| 44a.
UV+Visible Atmospheric Window:
See the blue line. You should have
a circle around the open (not black) areas in the part of the spectrum that
is roughly around
the
range of 0.3 - 0.7 μm
in the shortwave part of the spectrum.
WHY is this wavelength range referred to as an "atmospheric window
Think about what a regular glass window does (allows light to pass through
it.) Know that "light" is a general term sometimes used for
electromagnetic radiation or energy involving photons, whether the
radiation is UV or visible or even IR -- then proceed with an explanation
based on that. |
 |
|
44b.
IR Atmospheric Window:
See the red line. You should have a circle around the open (not black) areas in the part of
the spectrum that is roughly around 8 - 12
μm.
Note that there is one “spike” of
absorption that takes place right in the middle of the 8 - 12
μm IR atmospheric window at ~ 9.6 μm.. This
absorption is caused by the gas ozone. (See p 49 in SGC-E-Text and the caption
for Fig. 3-13)
Why is it so important ?